- High throughput
-
High throughput
One scanner can scan up to 60 slides per hour for a slide with 15x15mm tissue size. Each scanner has a slide capacity of 300 slides that can be continuously loaded, truly enabling fully automated nonstop scanning. Even if an error is detected it will indicate this to the user but continue scanning the next available slide, enabling high throughput digitization. - High quality images
-
High quality images
Our clinically validated scanner delivers high quality images by using a standardized and fully automated scanning process. Each slide is scanned at the equivalence of 40 times magnification (0.25 um/pixel). This results in sharp high resolution images which are vital for digitizing the primary diagnostic workflow and a key enabler for the use of image analysis algorithms. - Automated operation
-
Automated operation
All Philips scanners are designed for ‘load and walk away’ operation. Anyone in the lab can load slides into the scanner and then the digitization process will proceed automatically. This automated processing is supported by numerous algorithms for detection of tissue, barcode, and coverslip, as well as continuous auto-focusing algorithms used during scanning. - Multi-site harmonization and security
-
Multi-site harmonization and security
The multi-site setup is a common approach to creating a virtual network made up of multiple laboratories, allowing you to connect your team with a scalable solution and sharing histology data across an organization. Multiple sites can access each other’s images and exchange cases which enables workload balancing across locations. This enhances your ability to quickly reach specialists and sub-specialists for remote consultations. - Streamlining digital workflow to enhance user experience
-
Streamlining digital workflow to enhance user experience
IMS offers specialized tools for measurement, annotation, collaboration, and archive management. Review cases quickly using fast slide-to-slide transitions and confer on cases using single-click collaboration connection. Case details are presented with a digital slide tray, which organizes the slides like an analog slide tray. It includes case-related documents, grossing images and case-specific notes. This overview of slides can indicate which slides have been viewed, annotated and if additional slides have been ordered. Access role-based, user-specific case lists along with related patient data and notes. - Enhancing tissue analysis with a dedicated slide viewer
-
Enhancing tissue analysis with a dedicated slide viewer
Our dedicated slide viewer includes a rich set of tools for making annotations and accurate measurements, allowing pathologist to easily navigate tissue. Create a gallery of highlighted tissue features you can return to with the click of a button. Tag slides for follow-up in secondary workflows such as tumor board or panel discussions. Smart workflow algorithms include automatic image alignment, tissue detection, tissue presentation and single-click navigation. Shortcut keys help improve workflow and enhance productivity. - Enhancing real-time collaboration and case sharing
-
Enhancing real-time collaboration and case sharing
Connect with your colleagues anywhere in the world with our real-time collaboration tool. Share your slide viewing simultaneously in a manner that mimics a multi-headed microscope. Participants share control of the screen including annotation and measurement tools allowing them to collaborate on case analysis. - Improving the user experience and productivity
-
Improving the user experience and productivity
The Image Management System is your gateway to manage, review and analyze digitized slides. This next-generation software includes tools to optimize your daily caseload management, cases and images navigation and collaboration. Access whole slide images (WSI) in the feature-rich viewer in a streamlined, accessible diagnostic workflow. - Managing your workday just became easier
-
Managing your workday just became easier
IMS makes it possible to easily organize, review, manage and present digital cases. Facilitate organized personal worklists that are arranged and sorted according to your preference. Easily distinguish cases by status and case-related information such as patient name, number of slides per case and tissue type. Find the right case quickly with advanced search and filter functionality. - Flexible storage for digital pathology images
-
Flexible storage for digital pathology images
Flexible storage for digital pathology images Pathology image management can be tailored to meet diverse needs—whether through a customizable on-premise storage configuration or in combination with secure cloud-based archiving. The Philips IntelliSite Pathology Solution offers robust repository management with LIS interoperability, network flexibility, and seamless integration with existing systems. Cloud Data Services ensures scalable, cost-effective storage with instant access, long-term retention, and a foundation for AI-powered integrated diagnostics.** - Affordable total cost of ownership per digitized slide
-
Affordable total cost of ownership per digitized slide
The SG300 is designed to for medium to large laboratories that are running a traditional workflow with large batches of slides are processed for scanning at a few occasions per day. Offering high image quality, full automation and same first time right rate as the SG60, the SG300 enables high, clinical representative, throughput through it's scan speed, slide handling and non-rectangular ROI optimization. This results in n short turnaround times and a very low total cost of ownership (TCO/slide) as overnight scanning enables high utilization of the SG300. - 3D ready technology
-
3D ready technology
The Pathology Scanner Second Generation hardware solution prepared for multi-layer scanning.
High throughput

High throughput

High throughput
High quality images

High quality images

High quality images
Automated operation

Automated operation

Automated operation
Multi-site harmonization and security

Multi-site harmonization and security

Multi-site harmonization and security
Streamlining digital workflow to enhance user experience
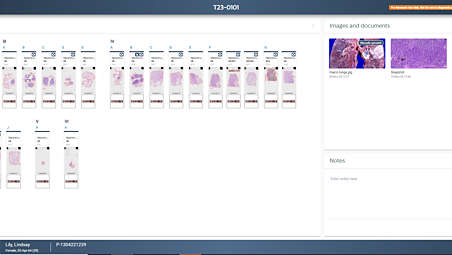
Streamlining digital workflow to enhance user experience

Streamlining digital workflow to enhance user experience
Enhancing tissue analysis with a dedicated slide viewer
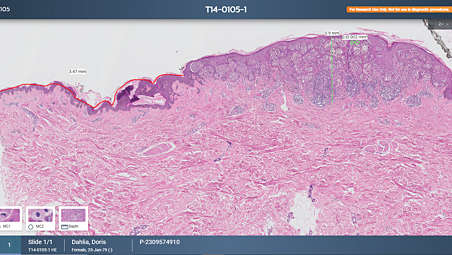
Enhancing tissue analysis with a dedicated slide viewer

Enhancing tissue analysis with a dedicated slide viewer
Enhancing real-time collaboration and case sharing
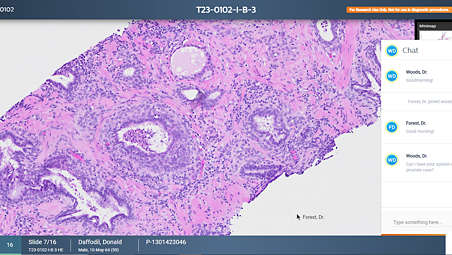
Enhancing real-time collaboration and case sharing

Enhancing real-time collaboration and case sharing
Improving the user experience and productivity
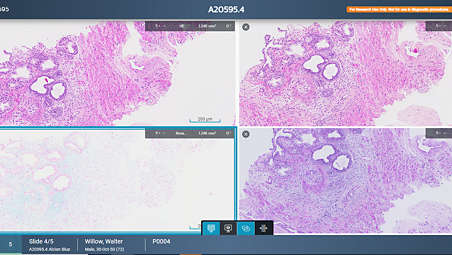
Improving the user experience and productivity

Improving the user experience and productivity
Managing your workday just became easier
Managing your workday just became easier

Managing your workday just became easier
Flexible storage for digital pathology images

Flexible storage for digital pathology images

Flexible storage for digital pathology images
Affordable total cost of ownership per digitized slide

Affordable total cost of ownership per digitized slide

Affordable total cost of ownership per digitized slide
3D ready technology
3D ready technology

3D ready technology
- High throughput
- High quality images
- Automated operation
- Multi-site harmonization and security
- High throughput
-
High throughput
One scanner can scan up to 60 slides per hour for a slide with 15x15mm tissue size. Each scanner has a slide capacity of 300 slides that can be continuously loaded, truly enabling fully automated nonstop scanning. Even if an error is detected it will indicate this to the user but continue scanning the next available slide, enabling high throughput digitization. - High quality images
-
High quality images
Our clinically validated scanner delivers high quality images by using a standardized and fully automated scanning process. Each slide is scanned at the equivalence of 40 times magnification (0.25 um/pixel). This results in sharp high resolution images which are vital for digitizing the primary diagnostic workflow and a key enabler for the use of image analysis algorithms. - Automated operation
-
Automated operation
All Philips scanners are designed for ‘load and walk away’ operation. Anyone in the lab can load slides into the scanner and then the digitization process will proceed automatically. This automated processing is supported by numerous algorithms for detection of tissue, barcode, and coverslip, as well as continuous auto-focusing algorithms used during scanning. - Multi-site harmonization and security
-
Multi-site harmonization and security
The multi-site setup is a common approach to creating a virtual network made up of multiple laboratories, allowing you to connect your team with a scalable solution and sharing histology data across an organization. Multiple sites can access each other’s images and exchange cases which enables workload balancing across locations. This enhances your ability to quickly reach specialists and sub-specialists for remote consultations. - Streamlining digital workflow to enhance user experience
-
Streamlining digital workflow to enhance user experience
IMS offers specialized tools for measurement, annotation, collaboration, and archive management. Review cases quickly using fast slide-to-slide transitions and confer on cases using single-click collaboration connection. Case details are presented with a digital slide tray, which organizes the slides like an analog slide tray. It includes case-related documents, grossing images and case-specific notes. This overview of slides can indicate which slides have been viewed, annotated and if additional slides have been ordered. Access role-based, user-specific case lists along with related patient data and notes. - Enhancing tissue analysis with a dedicated slide viewer
-
Enhancing tissue analysis with a dedicated slide viewer
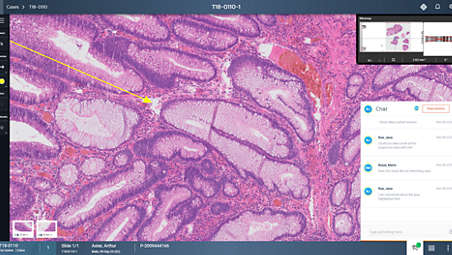
Our dedicated slide viewer includes a rich set of tools for making annotations and accurate measurements, allowing pathologist to easily navigate tissue. Create a gallery of highlighted tissue features you can return to with the click of a button. Tag slides for follow-up in secondary workflows such as tumor board or panel discussions. Smart workflow algorithms include automatic image alignment, tissue detection, tissue presentation and single-click navigation. Shortcut keys help improve workflow and enhance productivity. - Enhancing real-time collaboration and case sharing
-
Enhancing real-time collaboration and case sharing
Connect with your colleagues anywhere in the world with our real-time collaboration tool. Share your slide viewing simultaneously in a manner that mimics a multi-headed microscope. Participants share control of the screen including annotation and measurement tools allowing them to collaborate on case analysis. - Improving the user experience and productivity
-
Improving the user experience and productivity
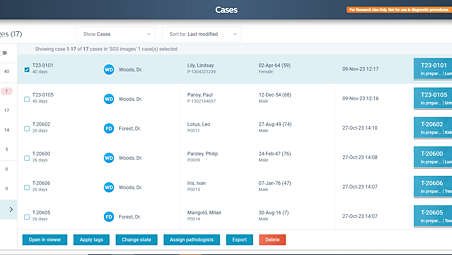
The Image Management System is your gateway to manage, review and analyze digitized slides. This next-generation software includes tools to optimize your daily caseload management, cases and images navigation and collaboration. Access whole slide images (WSI) in the feature-rich viewer in a streamlined, accessible diagnostic workflow. - Managing your workday just became easier
-
Managing your workday just became easier
IMS makes it possible to easily organize, review, manage and present digital cases. Facilitate organized personal worklists that are arranged and sorted according to your preference. Easily distinguish cases by status and case-related information such as patient name, number of slides per case and tissue type. Find the right case quickly with advanced search and filter functionality. - Flexible storage for digital pathology images
-
Flexible storage for digital pathology images
Flexible storage for digital pathology images Pathology image management can be tailored to meet diverse needs—whether through a customizable on-premise storage configuration or in combination with secure cloud-based archiving. The Philips IntelliSite Pathology Solution offers robust repository management with LIS interoperability, network flexibility, and seamless integration with existing systems. Cloud Data Services ensures scalable, cost-effective storage with instant access, long-term retention, and a foundation for AI-powered integrated diagnostics.** - Affordable total cost of ownership per digitized slide
-
Affordable total cost of ownership per digitized slide
The SG300 is designed to for medium to large laboratories that are running a traditional workflow with large batches of slides are processed for scanning at a few occasions per day. Offering high image quality, full automation and same first time right rate as the SG60, the SG300 enables high, clinical representative, throughput through it's scan speed, slide handling and non-rectangular ROI optimization. This results in n short turnaround times and a very low total cost of ownership (TCO/slide) as overnight scanning enables high utilization of the SG300. - 3D ready technology
-
3D ready technology
The Pathology Scanner Second Generation hardware solution prepared for multi-layer scanning.
High throughput

High throughput

High throughput
High quality images

High quality images

High quality images
Automated operation

Automated operation

Automated operation
Multi-site harmonization and security

Multi-site harmonization and security

Multi-site harmonization and security
Streamlining digital workflow to enhance user experience

Streamlining digital workflow to enhance user experience

Streamlining digital workflow to enhance user experience
Enhancing tissue analysis with a dedicated slide viewer

Enhancing tissue analysis with a dedicated slide viewer

Enhancing tissue analysis with a dedicated slide viewer
Enhancing real-time collaboration and case sharing

Enhancing real-time collaboration and case sharing

Enhancing real-time collaboration and case sharing
Improving the user experience and productivity

Improving the user experience and productivity

Improving the user experience and productivity
Managing your workday just became easier

Managing your workday just became easier

Managing your workday just became easier
Flexible storage for digital pathology images

Flexible storage for digital pathology images

Flexible storage for digital pathology images
Affordable total cost of ownership per digitized slide

Affordable total cost of ownership per digitized slide

Affordable total cost of ownership per digitized slide
3D ready technology

3D ready technology

3D ready technology
Documentation
-
Brochure (3)
-
Brochure
- Product Brochure (11.2 MB)
- Product Brochure (332.8 MB)
- Product Brochure (1008.6 kB)
-
Customer story (1)
-
Customer story
- Customer Story (240.9 kB)
-
Specification (1)
-
Specification
- Specification Sheet (67.1 MB)
-
Brochure (3)
-
Brochure
- Product Brochure (11.2 MB)
-
Customer story (1)
-
Customer story
- Customer Story (240.9 kB)
-
Brochure (3)
-
Brochure
- Product Brochure (11.2 MB)
- Product Brochure (332.8 MB)
- Product Brochure (1008.6 kB)
-
Customer story (1)
-
Customer story
- Customer Story (240.9 kB)
-
Specification (1)
-
Specification
- Specification Sheet (67.1 MB)
Specifications
- Pathology Scanner Second Generation
-
Pathology Scanner Second Generation Slide Capacity - 300
Scanning method - TDI line scanning
Focus method - Autofocus
Output format - iSyntax, DICOM JPEG* and DICOM JPEG XL*
Slide rack - Winlab LS-20/ Winlab LSM-20, Sakura 4768 20-slide basket (max. number of slides 20)
Operating temperature - 10 to 35°C
Dimensions (LxWxH in mm) doors closed - 680x950x675
Scan time (excluding handling and pre-scan per slide) - SG300 ≤ 45 seconds at 40x equivalent (15x15mm scan area)
Total scan time (including handling and pre-scan) per slide - SG300 ≤ 67 seconds at 40x equivalent (15x15mm scan area)
Average percentage of tissue detected and scanned - ≥ 99.5%
Power supply - 100-240 V AC, 50/60 Hz, 700 Watt
Magnification objective - NA of 0.75 plan Apo
Pixel size/ resolution - 0.25 μm/ pixel
Compliance to standards - EN IEC 61010-1:2010 /A1:2016, EN IEC 61010-2-101:2018, EN IEC 61326-1:2013 FCC Part 15, The IEC 6132
- The IEC 61326-2-6 (IVD), IEC 61326-1:2012 IEC 61326-2-6:2012
Barcode support - 1D code type: Code 39 with mod 43 checksum (ISO/IEC 16388:2007)
- Code 128 (ISO/IEC 15417:2007)
- 2D code type: Data Matrix ECC 200 - Code 39,
- Code 128 (ISO/IEC16022:2006) Recommended barcode type for all glass slides.
Relative humidity (no condensation) - Between 50% at 40 °C and 80% at 31 °C
Weight (kg) - 153
SG connectivity ports - Ethernet cable for 10GB (required CAT 6 Cable) / and 1GB/s network cable with RJ45
- connector (preferable CAT 6)
-
- Image Management System Review Application
-
Image Management System Review Application Minimal requirements of client computer to run the IMS viewer software - .
CPU - Intel Xeon E5-1620 v3 @ GHz or similar
RAM - 8 GB physical memory
Connectivity - 100 Mbit/s Ethernet
Video card - GPU Memory: 4GB GeForce GTX 1050 Ti or similar
- *Updating the graphics card driver on the client computer to the latest version is recommended.
-
- Software requirements on client computer
-
Software requirements on client computer Operating system - Microsoft Windows 8, 8.1 or 10-64 bit
Browser software supporting HTML 5 standard - 100 Mbit/s Ethernet
Connectivity - Chrome (recommended), Internet Explorer***
- *** Chrome is required for the feature Use my computer for image processing.
Video card - GPU Memory: 4GB
- GeForce GTX 1050 Ti or similar*
- *Updating the graphics card driver on the client computer to the latest version is recommended.
Other software - PDF viewer
- E-mail client
-
- Image Management System Application Server and Storage Software
-
Image Management System Application Server and Storage Software CPU - Dual socket Intel 6-core @ 2.3GHz
RAM - 32GB
Storage - Protection against a single disk failure, e.g. RAID configuration or data replication.
- For usage with 5 scanners or more: Flash Disks
-
- Pathology Scanner Second Generation
-
Pathology Scanner Second Generation Slide Capacity - 300
Scanning method - TDI line scanning
-
- Image Management System Review Application
-
Image Management System Review Application Minimal requirements of client computer to run the IMS viewer software - .
CPU - Intel Xeon E5-1620 v3 @ GHz or similar
-
- Pathology Scanner Second Generation
-
Pathology Scanner Second Generation Slide Capacity - 300
Scanning method - TDI line scanning
Focus method - Autofocus
Output format - iSyntax, DICOM JPEG* and DICOM JPEG XL*
Slide rack - Winlab LS-20/ Winlab LSM-20, Sakura 4768 20-slide basket (max. number of slides 20)
Operating temperature - 10 to 35°C
Dimensions (LxWxH in mm) doors closed - 680x950x675
Scan time (excluding handling and pre-scan per slide) - SG300 ≤ 45 seconds at 40x equivalent (15x15mm scan area)
Total scan time (including handling and pre-scan) per slide - SG300 ≤ 67 seconds at 40x equivalent (15x15mm scan area)
Average percentage of tissue detected and scanned - ≥ 99.5%
Power supply - 100-240 V AC, 50/60 Hz, 700 Watt
Magnification objective - NA of 0.75 plan Apo
Pixel size/ resolution - 0.25 μm/ pixel
Compliance to standards - EN IEC 61010-1:2010 /A1:2016, EN IEC 61010-2-101:2018, EN IEC 61326-1:2013 FCC Part 15, The IEC 6132
- The IEC 61326-2-6 (IVD), IEC 61326-1:2012 IEC 61326-2-6:2012
Barcode support - 1D code type: Code 39 with mod 43 checksum (ISO/IEC 16388:2007)
- Code 128 (ISO/IEC 15417:2007)
- 2D code type: Data Matrix ECC 200 - Code 39,
- Code 128 (ISO/IEC16022:2006) Recommended barcode type for all glass slides.
Relative humidity (no condensation) - Between 50% at 40 °C and 80% at 31 °C
Weight (kg) - 153
SG connectivity ports - Ethernet cable for 10GB (required CAT 6 Cable) / and 1GB/s network cable with RJ45
- connector (preferable CAT 6)
-
- Image Management System Review Application
-
Image Management System Review Application Minimal requirements of client computer to run the IMS viewer software - .
CPU - Intel Xeon E5-1620 v3 @ GHz or similar
RAM - 8 GB physical memory
Connectivity - 100 Mbit/s Ethernet
Video card - GPU Memory: 4GB GeForce GTX 1050 Ti or similar
- *Updating the graphics card driver on the client computer to the latest version is recommended.
-
- Software requirements on client computer
-
Software requirements on client computer Operating system - Microsoft Windows 8, 8.1 or 10-64 bit
Browser software supporting HTML 5 standard - 100 Mbit/s Ethernet
Connectivity - Chrome (recommended), Internet Explorer***
- *** Chrome is required for the feature Use my computer for image processing.
Video card - GPU Memory: 4GB
- GeForce GTX 1050 Ti or similar*
- *Updating the graphics card driver on the client computer to the latest version is recommended.
Other software - PDF viewer
- E-mail client
-
- Image Management System Application Server and Storage Software
-
Image Management System Application Server and Storage Software CPU - Dual socket Intel 6-core @ 2.3GHz
RAM - 32GB
Storage - Protection against a single disk failure, e.g. RAID configuration or data replication.
- For usage with 5 scanners or more: Flash Disks
-
- *Only with Philips Pathology Scanner SGi, not yet CE marked and not available for sale in the USA and certain other countries. Please contact your sales representative to ascertain availability in your country.
- **Third party claims from AWS, which may not apply in all markets and may be updated from time to time. The functionalities and benefits of the solution depend on customer-specific configuration and use. Please contact your local Philips representative for (market) availability.
- Philips IntelliSite Pathology Solution (PIPS) is in conformance with Regulation (EU) 201 7/746 of the European Parliament and of the Council of 5 April 2017 on In Vitro Diagnostics (IVDR).
- Not for viewing in the USA
